Influenza Diagnostic Market Size, Growth Forecast 2025-2032 | Industry Drivers, Key Players & Report
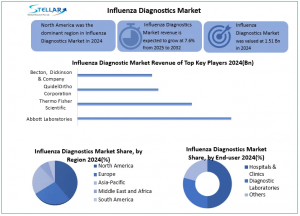
Influenza Diagnostic Market was estimated at USD 1.51 Bn. in 2024 is expected to grow at a CAGR of 7.6% from 2025 to 2032, reaching nearly USD 2.71 Bn. by 2032
Rising influenza cases and innovations in RIDTs, RT-PCR, and AI-powered testing are fueling rapid growth in North America diagnostics.”
WILMINGTON, DE, UNITED STATES, October 7, 2025 /EINPresswire.com/ -- Explore the US and North America Influenza Diagnostic Market, valued at USD 1.51 Bn in 2024 and growing at a CAGR of 7.6% to reach USD 2.71 Bn by 2032. Discover RIDTs, RT-PCR, molecular diagnostics, key players like Thermo Fisher, Quidel, Abbott, and top investment opportunities driving rapid, accurate influenza detection.— Navneet Kaur
Influenza Diagnostic Market Overview:
Influenza Diagnostic Market in US & North America is booming, driven by rising influenza cases, surging demand for RIDTs, and advanced molecular diagnostics like RT-PCR, TMA/TMABAS, and ELISA reagents. Leaders Abbott, Thermo Fisher, QuidelOrtho, and Becton Dickinson are accelerating growth with FDA-approved home kits, multiplex RT-PCR panels, and AI-powered testing, while government surveillance and post-COVID combo virus kits expand adoption across hospitals, clinics, labs, and at-home settings. With faster, accurate, and accessible influenza detection, North America emerges as a hotspot for high-return investment, market growth, and competitive innovation.
Influenza Diagnostic Market Set to Soar: Rapid Tests and RT-PCR Innovations Redefine US Flu Detection
The Influenza Diagnostic Market is booming as influenza causes 3–5 million serious illnesses and up to 650,000 deaths annually. Demand for rapid influenza diagnostic tests, RT-PCR testing, and molecular diagnostic tools is skyrocketing across hospitals, clinics, and point-of-care settings. Innovations like INAAT/TMABAS and FDA-approved home test kits are transforming US and North America flu diagnostics, driving faster, more accurate detection and reshaping the market.
👉 Access the full Research Description at: https://www.stellarmr.com/report/req_sample/influenza-diagnostic-market/2774
Influenza Diagnostic Market Transformed: FDA-Approved Home Kits and AI-Powered Flu Testing
The Influenza Diagnostic Market is witnessing a breakthrough with home-based flu testing powered by telehealth, AI symptom checkers, and smart diagnostic devices. FDA-approved home test kits now deliver results in under 30 minutes, often syncing with mobile apps for virtual doctor consultations and real-time prescriptions. This trend is transforming US and North America influenza diagnostics, unlocking a new era of fast, accurate, and patient-friendly influenza detection.
Influenza Diagnostic Market Struggles with RT-PCR Gaps and Rural Testing Risks
The Influenza Diagnostic Market faces a critical challenge: advanced RT-PCR influenza tests remain costly ($50–$150), requiring specialized equipment and trained staff, making them scarce in rural clinics and lower-income regions. While rapid antigen tests are cheaper, their accuracy can drop to 50–70%, raising the risk of misdiagnosis. To bridge this gap, expanding affordable, FDA-approved rapid and molecular test kits can improve US and North America influenza diagnostics and ensure timely, reliable detection across all healthcare settings.
Influenza Diagnostic Market Dominated by Test Kits, RT-PCR, and TMA Innovations
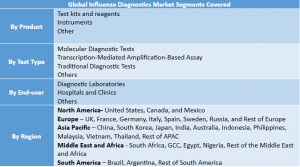
The Influenza Diagnostic Market is led by test kits and reagents (62% revenue in 2024), driven by high demand in hospitals, diagnostic labs, and at-home testing. Rapid antigen kits, RT-PCR, and ELISA reagents ensure consistent use, while FDA-approved home kits and government programs expand access. Molecular diagnostics, including TMA/TMABAS, dominate test types, fueled by post-COVID demand for combo kits detecting influenza, SARS-CoV-2, and RSV. These innovations are reshaping US and North America influenza diagnostics with faster, accurate, and accessible testing.
Innovation and Rapid Testing Fuel Growth in the US & North America Influenza Diagnostic Market
Strategic initiatives by key players, including development of innovative test kits and molecular diagnostics for public health applications, are accelerating growth in the US and North America Influenza Diagnostic Market.
Rapid Influenza Diagnostic Tests (RIDTs) are driving growth in the US and North America Influenza Diagnostic Market with fast, point-of-care results.
Major Product Launches and RT-PCR Innovations Propel US & North America Influenza Diagnostic Market
April 2, 2024 Thermo Fisher Scientific:
Thermo Fisher upgraded its TaqPath multiplex RT-PCR assay covering 20 targets, including influenza strains, boosting its position in the US and North America Influenza Diagnostic Market.
February 14, 2024 QuidelOrtho Corporation:
QuidelOrtho launched the enhanced Sofia 2 Flu + COVID Antigen FIA in North America, strengthening its presence in the US and North America Influenza Diagnostic Market.
👉 Access the full Research Description at: https://www.stellarmr.com/report/req_sample/influenza-diagnostic-market/2774
Influenza Diagnostic Market Booms in US & Canada with Large-Scale Molecular Testing
The North America Influenza Diagnostic Market led in 2024 and is poised to maintain dominance through 2032, driven by robust healthcare infrastructure, high diagnostic sensitivity, and government influenza surveillance programs. Extensive hospitals, clinics, and laboratories in the US and Canada enable widespread use of highly sensitive molecular assays, including RT-PCR, supporting rapid, accurate, and large-scale influenza detection, making this region a hotspot for innovation and market growth.
Global Leaders Drive Innovation in the US & North America Influenza Diagnostic Market
The Influenza Diagnostic Market is dominated by global leaders like Abbott Laboratories, Thermo Fisher Scientific, Quidel Corporation, and Becton Dickinson, controlling over 60% of the 2024 market. These companies leverage comprehensive test kit portfolios, strong brand recognition, and robust distribution channels, while investing heavily in R&D for rapid and multiplex molecular diagnostics. Notably, on February 14, 2024, QuidelOrtho launched its enhanced Sofia 2 Flu + COVID Antigen FIA in North America, strengthening its footprint in the US and North America Influenza Diagnostic Market and pushing innovation in point-of-care testing.
Influenza Diagnostic Market Key Players:
North America
Abbott Laboratories (United States)
Thermo Fisher Scientific (United States)
QuidelOrtho Corporation (United States)
Becton, Dickinson and Company – BD (United States)
Hologic Inc. (United States)
Cepheid (United States)
Meridian Bioscience (United States)
BioFire Diagnostics (United States)
GenMark Diagnostics (United States)
Luminex Corporation (United States)
Europe
Roche Diagnostics (Switzerland)
Siemens Healthineers (Germany)
bioMérieux SA (France)
Randox Laboratories (United Kingdom)
Eurofins Scientific (Luxembourg)
DRG Diagnostics (Germany)
Abingdon Health (United Kingdom)
Omega Diagnostics (United Kingdom)
Fast Track Diagnostics (Luxembourg)
Molbio Diagnostics GmbH (Germany)
Asia-Pacific
Sysmex Corporation (Japan)
Fujirebio Inc. (Japan)
Seegene Inc. (South Korea)
SD Biosensor (South Korea)
Mylab Discovery Solutions (India)
Analyst Perspective:
The US and North America Influenza Diagnostic Market is booming, fueled by rising influenza cases, demand for RIDTs and molecular diagnostics, and innovations from Abbott, Thermo Fisher, QuidelOrtho, and BD. FDA-approved home kits, multiplex RT-PCR, and AI-driven solutions, alongside government surveillance, are driving faster, accurate, and widely accessible influenza detection, making North America a hotspot for growth and high-return investment.
FAQ:
Q1: Why read this Influenza Diagnostic Market report?
A1: Discover market size, CAGR, key players, and trends like RIDTs, RT-PCR, and FDA-approved home kits.
Q2: How does this report aid business decisions?
A2: Uncover investment opportunities, competitive strategies, and innovations in US and North America influenza diagnostics.
Q3: What drives growth in this market?
A3: Rising influenza cases, rapid and molecular diagnostics, AI testing, and government surveillance programs.
Maximize Market Research is launching a subscription model for data and analysis in the
Dental Materials market https://www.mmrstatistics.com/markets/512/topic/657/healthcare
Related Reports:
Medical Image Analysis Software Market: https://www.stellarmr.com/report/medical-image-analysis-software-market/2836
Radiotherapy Market: https://www.stellarmr.com/report/radiotherapy-market/2834
Paronychia Treatment Market: https://www.stellarmr.com/report/paronychia-treatment-market/2825
Ophthalmic Disease Therapeutics Market: https://www.stellarmr.com/report/ophthalmic-disease-therapeutics-market/2824
Vulvar Cancer Market: https://www.stellarmr.com/report/vulvar-cancer-market/2812
About Stellar Market Research:
Stellar Market Research is a multifaceted market research and consulting company with professionals from several industries. Some of the industries we cover include medical devices, pharmaceutical manufacturers, science and engineering, electronic components, industrial equipment, technology and communication, cars and automobiles, chemical products and substances, general merchandise, beverages, personal care, and automated systems. To mention a few, we provide market-verified industry estimations, technical trend analysis, crucial market research, strategic advice, competition analysis, production and demand analysis, and client impact studies.
Lumawant Godage
Stellar Market Research
+ +91 9607365656
email us here
Visit us on social media:
LinkedIn
Instagram
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.